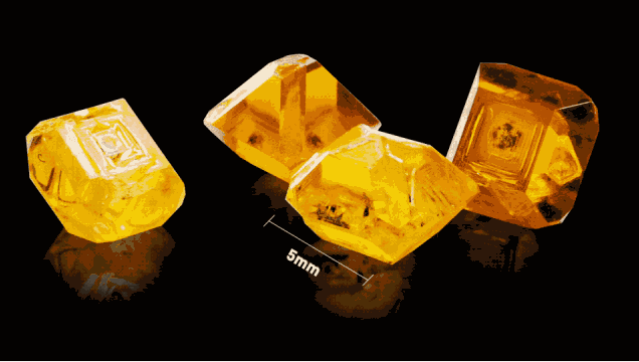
There are two diamonds here, the left one is a natural diamond that has been formed over the millions of years on the ground, and the right one is a synthetic diamond that took only three or four days to synthesize with mechanical equipment.

What is the difference between these two diamonds? How can I synthesize a high quality synthetic diamond?
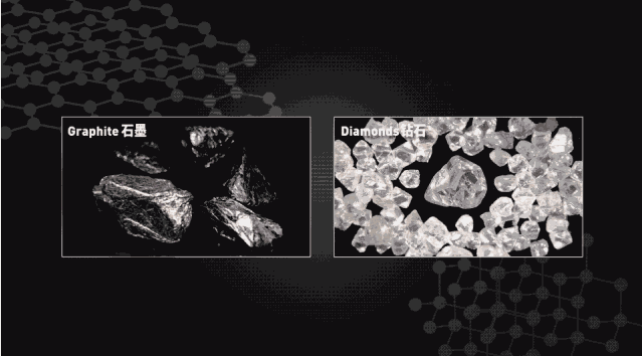
Diamonds, also known as diamonds, are made up of carbon atoms, like graphite, the main component of pencil lead. However, the difference in structure allows graphite to rank only at the lowest level 1 in the Mohs hardness class and diamonds at the highest 10 levels.
The key to the formation of different structures between carbon atoms is pressure and temperature.
It can be seen from this carbon phase diagram that under different pressure and temperature conditions, carbon atoms will become various forms such as graphite, diamonds, and liquids.
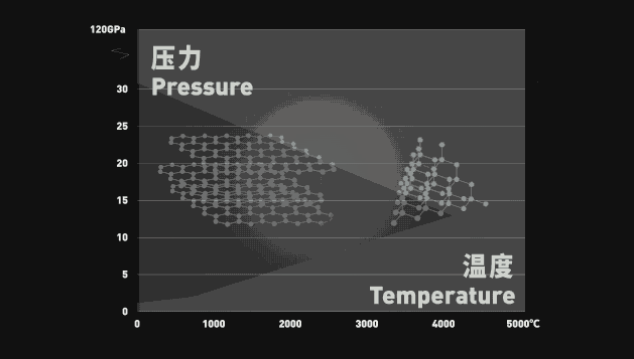
Where GPa is the pressure unit, 1GPa is equivalent to the power of a 10 ton African elephant stepping on your little toe.

This interval is the condition for the formation of natural diamonds.
In the underground 100~300km, about 4.5~6GPa, 1000~1500°C, the carbon element will form a diamond after two or three million years of extrusion, and finally it will be brought to the surface by volcanic activity.
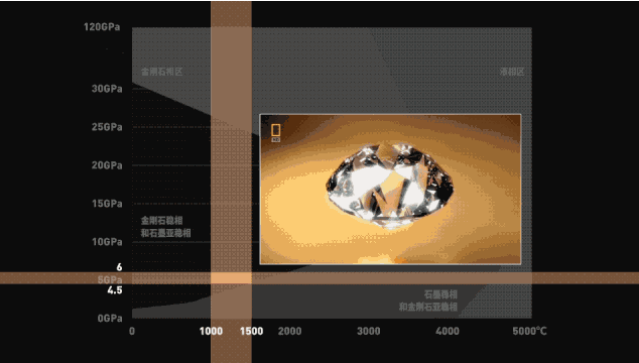
Therefore, diamonds can be synthesized by artificially creating corresponding pressure and temperature conditions based on the carbon phase diagram.
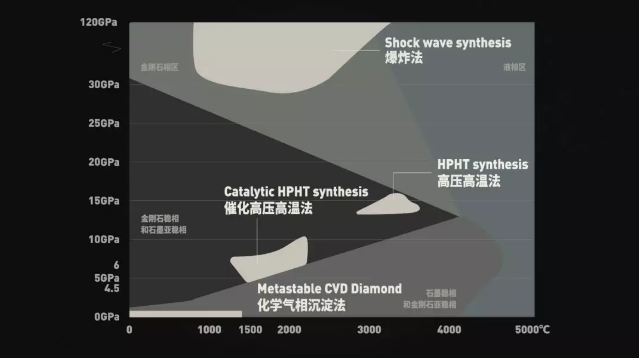
The simplest and most rude is the explosion method.
Mixing TNT with carbon atom and RDX with very high bombardment pressure, detonating in steel thick-walled tank, producing high temperature above 1000 °C and ultra-high pressure of 30 GPa, a part of carbon atoms in TNT will be polymerized into Diamonds of 5~15nm size are called diamond powder.
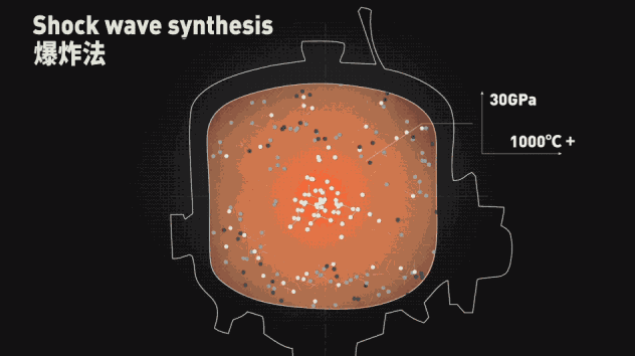
Diamond powder cannot be used directly as jewelry, but large-grain diamonds can be produced by high-pressure high-temperature methods.
This is a six-sided top press consisting of six top hammers with three axes at 90° and a growth chamber for synthetic diamonds inside.
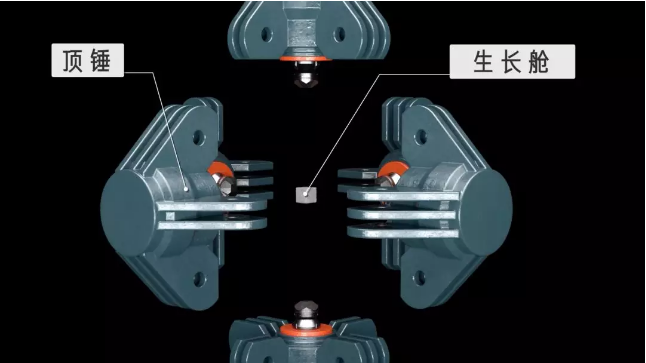
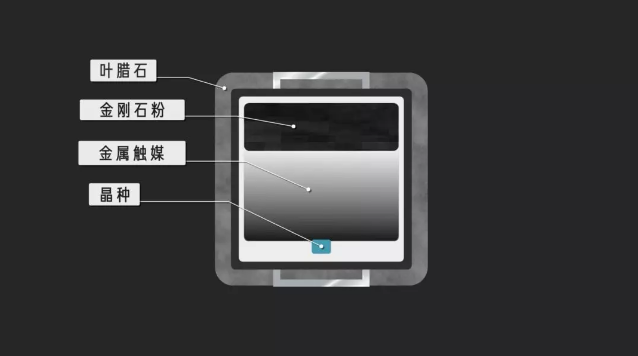
Outside the growth chamber is pyrophyllite, which conducts pressure well.
The inside is mainly divided into two layers, the upper layer is diamond powder, the lower layer is metal catalyst, generally nickel-iron, which plays a catalytic role to accelerate the speed of diamond synthesis. There is a natural or artificial diamond of less than 1mm as a seed crystal at the bottom.
At the time of starting, the top hammer is pressed inward to provide a high pressure of 5~6GPa, and the carbon heating rod is heated by conduction to make the temperature of the growth chamber reach above 1200°C, and a temperature difference of 20~50°C is created above and below.
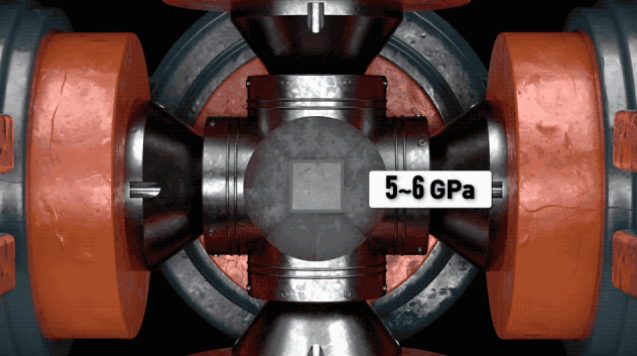
At this time, the metal catalyst will melt, and the diamond powder will dissolve into the metal catalyst to become free carbon atoms, and the carbon atom concentration in the high temperature region will be higher than that in the low temperature region.
In order to achieve concentration equilibrium, the carbon atoms in the high temperature zone will diffuse into the low temperature zone and precipitate on the seed crystals.
A synthetic diamond of about 5mm can be harvested in a week.

In addition to the six-face press, there are two sides and a split top press for synthetic diamonds.

The size of a diamond can be measured in carats, and 1 carat is equal to 0.2 grams.
The traditional high-pressure high-temperature method synthesizes a small number of diamonds, and the metal catalyst will leave a residue in the diamond. A very small amount of nitrogen in the machine will be mixed into the diamond, making the diamond yellow.

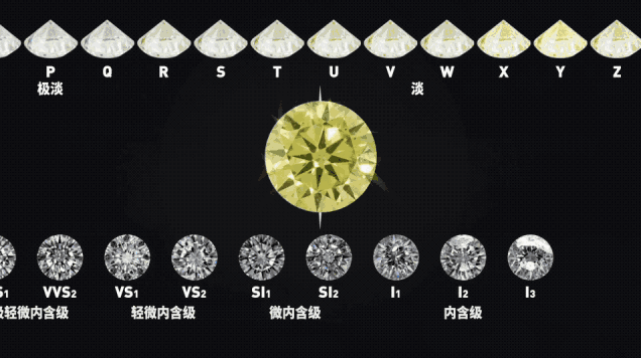
According to the diamond's 4C standard, this diamond is usually at the low end and can only be used for industrial purposes, making drills, knives, grinding discs, etc.
In contrast, chemical vapor deposition can greatly improve these defects.
This method also requires seed crystals, after mechanical polishing, acid treatment, etc. to remove surface impurities.
Place it in a vacuum chamber with only one tenth of an atmosphere, inject methane gas and hydrogen, and heat it to 800~1000 °C by microwave or the like.

At this time, methane will decompose carbon atoms, form a plasma, land on the seed crystals at the bottom, precipitate crystals, and grow at a rate of 0.006 mm per hour. Within two days, a graphite surrounded by graphite will be obtained. diamond.

This method of synthesizing diamonds can achieve the color and purity of high-grade natural diamonds, and the number of carats is also higher.
In 2017, the University of Augsburg, Germany, produced a diamond with a diameter of 92mm and 155 carats.
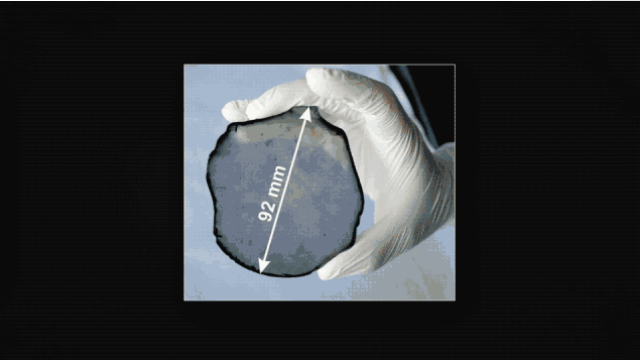
Synthetic diamonds are also genuine diamonds with the same composition and structure as natural diamonds.
The two can only be distinguished in a very special way. For example, under the cathodoluminometer, the growth texture of the former is geometric, while the latter is in the form of a ring.

Since 2006, jewellery organizations such as GIA have begun to provide identification services and issue certificates, mainly to prevent the sale of natural diamonds from synthetic diamonds with lower prices.

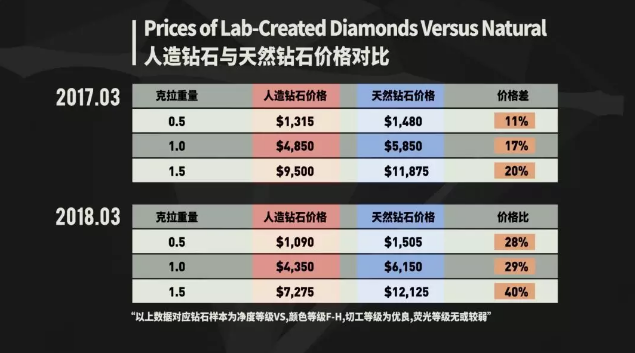
In 2017, the average selling price of synthetic diamonds was only 10-20% cheaper than natural diamonds. After one year, the gap has been extended to 30-40%.
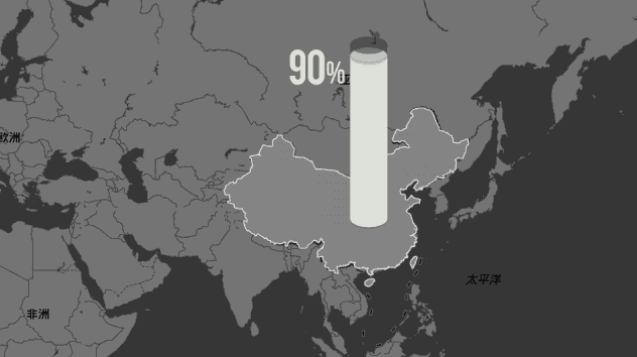
And this gap will grow bigger and bigger because the Chinese are coming.
China's industrial grade synthetic diamonds account for more than 90% of the world's annual production and are concentrated in three companies in Henan Province.
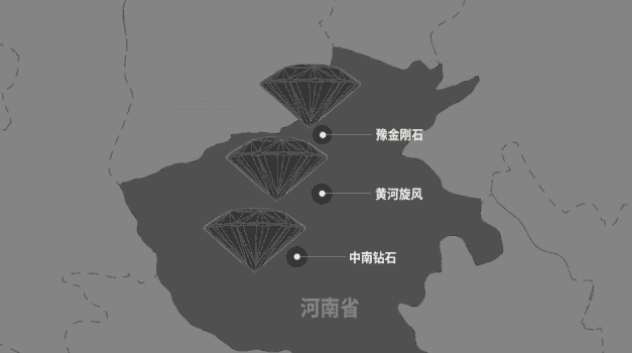
However, in the case of Henan diamond, in addition to the traditional industrial grade diamond, the annual production of colorless gem-quality diamonds can reach 2 to 3 million carats.
On the technical strength, China's annual import of 10 million carats of gem-quality diamonds can soon be produced by a province in Henan, but it depends on whether you are willing to buy.

Kids Wallpaper,Waterproof Pvc Vinyl Wallcovering,Pvc Morden Wallpaper,Non Woven Kids Wallpaper
SHAOXING VIGOUR DECORATION MATERIAL CO.,LTD , https://www.vigourwallpaper.com